Introduction to Cardiac Physiology
The human heart operates as a dynamic pump, adapting to constantly changing circulatory demands. At the core of this adaptability lies the pressure-volume relationship, which reflects how the ventricles fill and eject blood during each heartbeat. Among these relationships, two critical parameters — preload and the end-diastolic pressure-volume relationship (EDPVR) — define the mechanical behavior of the ventricle during diastole. Understanding their interplay helps clinicians and researchers interpret cardiac performance in both physiological and pathological states.
What is Preload?
Preload refers to the initial stretching of the cardiac myocytes at the end of diastole, just before contraction begins. It represents the ventricular filling volume or pressure and is directly influenced by venous return and ventricular compliance.
From a physiological standpoint, preload is often approximated by the end-diastolic volume (EDV) or end-diastolic pressure (EDP). The Frank-Starling mechanism describes how an increase in preload enhances stroke volume — up to an optimal limit — by improving sarcomere alignment and cross-bridge formation.
Factors Influencing Preload
- Venous Return – The volume of blood returning to the heart significantly affects preload. Increased venous tone or blood volume enhances filling.
- Ventricular Compliance – A more compliant ventricle can accommodate a larger volume with less pressure rise.
- Atrial Contraction – Contributes roughly 20% of ventricular filling, especially in stiff ventricles.
- Pericardial Pressure and Intrathoracic Pressure – Changes during respiration or mechanical ventilation can alter preload by affecting venous return.
Clinical Indicators of Preload
Clinicians often estimate preload using measurable pressures:
- Central Venous Pressure (CVP) for the right heart
- Pulmonary Capillary Wedge Pressure (PCWP) for the left heart
However, these are indirect measures and can be influenced by ventricular compliance, making direct interpretation challenging without considering EDPVR.
Understanding the End-Diastolic Pressure-Volume Relationship (EDPVR)
The EDPVR describes the relationship between ventricular pressure and volume at the end of diastole — a point when the ventricle is filled but not yet contracting. It reflects the passive mechanical properties of the myocardium, primarily determined by myocardial stiffness and chamber geometry.
The Pressure-Volume Loop Explained
The cardiac pressure-volume (PV) loop illustrates the heart’s mechanical cycle:
- Filling Phase (EDPVR segment) – Pressure rises slowly as volume increases.
- Isovolumetric Contraction – Volume constant; pressure sharply rises.
- Ejection Phase – Blood is expelled; volume decreases.
- Isovolumetric Relaxation – Pressure drops before the next filling phase.
The EDPVR defines the lower boundary of this loop, showing how pressure changes as the ventricle fills — essentially a measure of diastolic stiffness.
Mathematical Representation of EDPVR
The EDPVR is typically nonlinear, often represented by the equation:
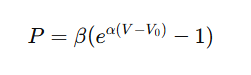
where:
- P = End-diastolic pressure
- V = Volume
- α = Stiffness constant
- β = Scaling factor
- V₀ = Unstressed volume
This exponential nature shows that small increases in volume cause large increases in pressure when the ventricle becomes stiff — a crucial insight in diastolic dysfunction.
The Relationship Between Preload and EDPVR
Preload and EDPVR are tightly linked. Preload determines how much the ventricle fills, while EDPVR defines how the ventricle reacts to that filling. In simple terms:
- Preload = Filling Volume/Pressure
- EDPVR = Passive stiffness relationship
At a given EDPVR, an increase in preload means the ventricle fills more, moving rightward along the curve — increasing end-diastolic volume (EDV) and pressure (EDP).
If the ventricle becomes stiffer, the EDPVR curve shifts upward and leftward, meaning for the same preload, end-diastolic pressure is higher, reflecting reduced compliance.
Impact of Ventricular Compliance on EDPVR
Ventricular compliance is the inverse of stiffness. When compliance decreases (as in hypertrophy or fibrosis), the EDPVR becomes steeper. Thus, even small increases in preload lead to large pressure rises — limiting filling and cardiac output.
Conversely, in highly compliant ventricles (like in athletes), preload can increase substantially with only minimal pressure change.
Clinical Relevance of the Preload–EDPVR Relationship
Understanding how preload interacts with EDPVR is not merely an academic concept — it’s fundamental in diagnosing and managing cardiovascular conditions. Clinicians often use this relationship to assess ventricular performance, fluid responsiveness, and diastolic function in critically ill patients.
Preload Responsiveness in Critical Care
In critical care medicine, determining whether a patient will benefit from fluid administration is vital. The Frank-Starling mechanism explains that when preload increases, stroke volume rises — but only up to a point.
Once the heart reaches the steep portion of the EDPVR curve, further fluid addition causes pressure to increase disproportionately compared to volume. This leads to pulmonary congestion without improving cardiac output — a hallmark of fluid overload.
Techniques to assess this include:
- Dynamic indices like pulse pressure variation (PPV) and stroke volume variation (SVV)
- Passive leg raising test, which transiently increases venous return to evaluate preload responsiveness
By referencing EDPVR behavior, clinicians can predict fluid responsiveness more accurately than by static measures like CVP.
Heart Failure and Diastolic Dysfunction
In heart failure with preserved ejection fraction (HFpEF), the EDPVR shifts upward and leftward. This shift indicates increased ventricular stiffness, so even normal volumes produce abnormally high filling pressures.
Patients experience pulmonary congestion, dyspnea, and exercise intolerance despite a seemingly normal ejection fraction. Conversely, in systolic heart failure (HFrEF), EDPVR may shift rightward due to ventricular dilation and reduced stiffness, increasing preload tolerance but reducing contractile force.
Thus, the relationship between preload and EDPVR provides a mechanistic framework to interpret hemodynamic abnormalities in both heart failure subtypes.
Experimental and Imaging Assessment
Quantifying preload and EDPVR requires precise tools. Modern techniques enable noninvasive or minimally invasive evaluation of these relationships, improving diagnostic accuracy.
Pressure-Volume Catheterization
This is the gold standard for measuring real-time pressure-volume loops. It involves a conductance catheter inserted into the ventricle to record instantaneous pressure and volume data. From these measurements, the EDPVR curve can be derived, offering direct insight into ventricular compliance.
Cardiac MRI
Cardiac magnetic resonance imaging (MRI) offers detailed structural and functional information. Using advanced modeling, MRI can derive diastolic pressure-volume curves and measure myocardial fibrosis — one of the primary determinants of increased stiffness.
Therapeutic Implications
Understanding how preload interacts with EDPVR guides a wide range of clinical interventions — from fluid management to pharmacologic therapy and mechanical circulatory support.
Pharmacological Interventions
- Diuretics – Reduce preload by decreasing blood volume, relieving congestion in stiff ventricles (common in HFpEF).
- Vasodilators – Lower venous tone, reducing venous return and end-diastolic pressure.
- Inotropes – Enhance contractility, shifting the end-systolic pressure-volume relationship (ESPVR) upward, indirectly improving stroke volume for a given preload.
- Beta-blockers and ACE inhibitors – Improve long-term compliance by reducing myocardial remodeling and stiffness.
The goal is to balance preload optimization with ventricular compliance, ensuring adequate cardiac output without excessive filling pressures.
Mechanical Support Devices
Mechanical circulatory supports like LVADs (Left Ventricular Assist Devices) and ECMO (Extracorporeal Membrane Oxygenation) significantly alter the preload-EDPVR dynamics:
- LVADs unload the ventricle, reducing end-diastolic volume and pressure, thus flattening the EDPVR curve.
- ECMO can increase afterload and preload depending on configuration, requiring careful volume management to prevent ventricular distension.
These insights are essential for tailoring therapy in patients with advanced heart failure or post-cardiac surgery.
Future Directions in Research
Emerging research focuses on developing personalized models of ventricular mechanics that integrate preload and EDPVR data. Using machine learning and computational modeling, scientists aim to predict individual cardiac responses under varying conditions, paving the way for precision cardiology.
Additionally, titin modulation therapies and gene editing targeting extracellular matrix components hold promise in modifying diastolic stiffness — potentially shifting the EDPVR in beneficial ways.
For further reading on EDPVR modeling and clinical implications, visit American Heart Association Journals.
FAQs About Preload and EDPVR
What is the difference between preload and EDPVR?
Preload refers to the ventricular filling condition (volume or pressure), while EDPVR describes how the ventricle’s pressure changes in response to that filling — reflecting passive stiffness.
How does preload affect EDPVR?
Increased preload moves the operating point rightward along the EDPVR curve. If the ventricle is compliant, pressure rises minimally; if stiff, pressure rises sharply.
What causes the EDPVR to shift upward?
Conditions like myocardial hypertrophy, fibrosis, or ischemia increase stiffness, shifting the curve upward and leftward, leading to higher diastolic pressures for the same volume.
How is EDPVR used clinically?
It helps assess diastolic function, heart failure type, and fluid responsiveness. Clinicians use derived parameters to decide fluid therapy and optimize preload.
Can EDPVR be measured noninvasively?
Yes. Echocardiographic and MRI-based techniques estimate diastolic stiffness and pressure-volume behavior without invasive catheters.
Why is understanding preload-EDPVR important in heart failure?
Because it helps differentiate HFpEF (stiff ventricle, high pressure) from HFrEF (dilated ventricle, low pressure) — guiding precise therapy.
Conclusion
The relationship between preload and EDPVR lies at the heart of cardiac physiology. Preload determines how much the ventricle fills, while EDPVR defines how the ventricle tolerates that filling — a delicate balance between volume and pressure.
In healthy hearts, increased preload enhances cardiac output efficiently. But in stiff or diseased ventricles, even minor volume changes can cause dramatic pressure rises, leading to symptoms of heart failure.
By integrating this relationship into clinical assessment, clinicians can make better-informed decisions regarding fluid management, medication, and device therapy — ultimately improving patient outcomes.
