Introduction to Parallel Conductance in Cardiac Physiology
In the world of cardiovascular research, accurate measurement of left ventricular volume is critical for understanding cardiac function. One of the most widely used tools is the conductance catheter, which provides real-time pressure-volume (PV) loop data. However, the raw signals obtained aren’t always reliable due to interference from surrounding tissues. This is where parallel conductance correction plays a vital role.
By compensating for unwanted electrical conductance outside the blood pool, parallel conductance correction ensures that PV loops truly reflect ventricular volume and function. Without this correction, data can be misleading, impacting both research outcomes and clinical interpretations.
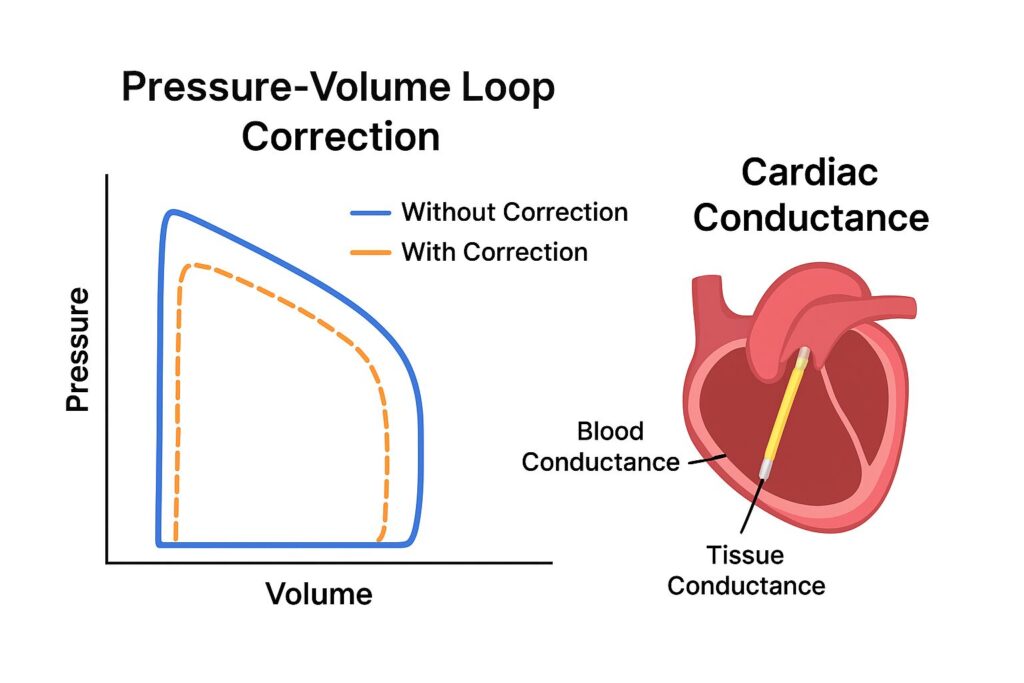
The Role of Conductance in Pressure-Volume Loop Analysis
Conductance catheters measure the electrical resistance across the ventricular chamber. Since blood is a good conductor, changes in volume can be tracked through conductance. When plotted against pressure, these data points form the iconic pressure-volume loops — a cornerstone in cardiac physiology.
Yet, the heart doesn’t exist in isolation. Muscle tissue, chest structures, and even lung conductivity interfere with readings. This interference is what scientists call parallel conductance (Gp). Correcting for Gp is therefore essential for extracting meaningful results.
Why Parallel Conductance Correction is Essential
If uncorrected, parallel conductance causes significant overestimation of ventricular volumes. For example, a PV loop may suggest a heart chamber is larger than it truly is, leading to false conclusions about systolic and diastolic function.
By applying correction techniques, researchers can isolate the “true blood conductance” and remove confounding variables. The result? More accurate assessments of contractility, compliance, stroke volume, and cardiac output.
Basics of Pressure-Volume Catheterization
To fully understand parallel conductance correction, one must first grasp the fundamentals of PV catheterization.
The Conductance Catheter Method
A conductance catheter is inserted into the ventricle, typically the left ventricle, through a minimally invasive procedure. The catheter contains electrodes that measure both pressure and conductance at multiple segments.
Measuring Left Ventricular Volume
The total conductance measured consists of two parts:
- Blood conductance (Gb) → representing the actual ventricular blood volume.
- Parallel conductance (Gp) → representing surrounding tissue conductivity.
Since the catheter records Gb + Gp, separating the two is essential for accuracy.
What is Parallel Conductance?
Sources of Parallel Conductance
Parallel conductance originates from structures outside the blood pool, such as the myocardium, lungs, and chest wall. These tissues have their own electrical properties and contribute to the conductance signal detected by the catheter.
The Impact of Tissue and Surrounding Structures
Because the catheter cannot distinguish between blood and tissue conductance, the measurement is inflated. For instance, in hypertrophied hearts, thickened myocardium may contribute disproportionately to parallel conductance, further complicating volume estimation.
The Need for Parallel Conductance Correction
Errors Without Correction
If parallel conductance isn’t accounted for, researchers may overestimate end-diastolic and end-systolic volumes. This miscalculation trickles down into derived parameters such as ejection fraction, stroke work, and ventricular elastance — leading to flawed conclusions.
Improving Accuracy in Volume Measurement
Correcting for parallel conductance ensures that PV loop-derived data reflect actual physiological conditions. With correction, cardiac performance metrics become reliable, making the results invaluable for both preclinical and translational research.
Techniques for Parallel Conductance Correction
The Saline Dilution Method
One of the most widely adopted techniques for parallel conductance correction is the saline dilution method.
Step-by-Step Process
- Inject a small bolus of hypertonic saline (usually 5% NaCl) into the bloodstream.
- The saline alters blood conductivity without affecting myocardial tissue conductivity.
- The shift in conductance is used to isolate and calculate Gp.
- Corrected volume measurements are obtained by subtracting Gp from total conductance.
Advantages and Limitations
- Advantages: Relatively simple, reproducible, and widely validated.
- Limitations: Can cause transient hemodynamic changes, not ideal for unstable patients, and requires precise timing.
Hypertonic Saline Bolus Technique
This approach builds on the saline dilution method but uses different concentrations and volumes of saline bolus. It is considered the gold standard for many experimental setups but requires careful calibration to avoid overcorrection.
Alternative Calibration Approaches
Researchers have explored alternative strategies, such as echocardiography-assisted calibration and MRI-based validation. These hybrid methods reduce reliance on invasive corrections but are often more resource-intensive.
Mathematical Principles Behind Correction
Conductance Equation Explained
The general conductance equation is:
G = α × (Gb + Gp)
Where:
- G = measured conductance
- Gb = blood conductance
- Gp = parallel conductance
- α = calibration constant
By determining Gp using saline dilution, researchers can mathematically isolate Gb for accurate volume estimation.
Volume Calibration Curves
Calibration involves plotting measured conductance against known volumes, generating a linear relationship. Once corrected for Gp, this curve yields precise left ventricular volume data across cardiac cycles.
Practical Applications in Cardiology Research
Heart Failure Studies
Accurate PV loop data are essential for understanding the progression of heart failure. Parallel conductance correction allows researchers to evaluate systolic dysfunction, diastolic stiffness, and contractile reserve.
Myocardial Contractility Assessment
Corrected conductance enables precise measurement of load-independent indices like end-systolic elastance (Ees), which reflect true myocardial contractility independent of preload and afterload conditions.
Common Challenges and Pitfalls
Experimental Variability
Factors such as catheter placement, blood conductivity changes, and inter-animal variability can impact correction accuracy. Standardizing procedures helps minimize these discrepancies.
Signal Artifacts
Electrical interference, respiratory motion, and arrhythmias may introduce noise into conductance recordings. Filtering techniques and repeated calibrations are often necessary.
Modern Advances in Parallel Conductance Correction
Automated Software Tools
Recent software advancements allow real-time correction of parallel conductance, reducing manual errors and saving time during experiments.
Integration with Imaging Techniques
Combining PV catheterization with imaging modalities like MRI or 3D echocardiography enhances validation and minimizes uncertainties in correction.
FAQs on Parallel Conductance Correction
Q1: Why is parallel conductance correction important in PV loop studies?
Because it removes background tissue signals, ensuring volume measurements truly reflect ventricular blood volume.
Q2: What happens if I skip parallel conductance correction?
You risk overestimating ventricular volumes, which leads to misleading conclusions about cardiac function.
Q3: Is the saline dilution method safe?
Yes, in most controlled research environments, but it may cause transient hemodynamic changes.
Q4: Does parallel conductance vary with species?
Yes. Small animals have proportionally higher Gp due to tissue thickness relative to ventricular volume.
Q5: What are the latest alternatives to saline dilution?
AI-driven corrections, MRI integration, and automated software-based calibrations are emerging as modern solutions.
Conclusion
Parallel conductance correction is a cornerstone of accurate cardiac physiology research. By separating true blood volume from tissue conductance, researchers obtain reliable PV loop data that inform everything from heart failure studies to drug development. While traditional saline-based methods remain the standard, emerging AI-driven and imaging-assisted approaches promise a future of faster, safer, and more precise corrections.
